Post COVID-19 – effective treatment and rehabilitation
A systematic review and assessment of medical and economic aspects
Background
SBU was commissioned by the Swedish Government to evaluate the scientific evidence for care for patients with post COVID-19 (long-term symptoms or sequelae of the disease COVID-19).
Aim
The aim was to summarize published scientific articles addressing the following research question: Which treatments are effective for post COVID-19?
Method
A systematic review conducted in accordance with THE PRISMA statement. The protocol is registered in Prospero. The certainty of evidence was assessed with GRADE.
Inclusion criteria
PICO
Population – 1. Patients with post COVID-19 condition according to WHO´s definition (individuals with persistent or new symptoms after 3 months from the initial onset of COVID-19 that last for at least 2 months and cannot be explained by an alternative diagnosis). 2. Patients who did not meet the criteria according to WHO´s definition (but had early symptoms, treatment started after infection clearance and were followed up at least three months).
Interventions – Treatment or rehabilitation for long-term symptoms.
Comparison – No treatment or other treatment.
Outcomes – All outcomes related to post COVID-19 (long-term symptoms or sequelae of the disease COVID-19).
Study design – RCT and non-randomised controlled trials. Observational and qualitative studies, as well as case studies, were excluded.
Search period: From April 26 2021, then weekly. Final search June 1 2022.
Databases searched: Every week, an information specialist searched the database Medline (OvidSP) via Alerts. Every month, five additional databases were searched: Cinahl (Ebsco), PsycINFO (Ebsco), Cochrane Library (Wiley), Embase (embase.com) and WHO: Global literature on coronavirus disease. Reference lists and citations for relevant primary studies and reviews were also screened.
The project group also continuously tracked the following COVID-19 specific resources:
- COVID-NMA
- Cochrane Rehabilitation
Patient involvement: No
Results
Evidence map: Post COVID-19 – effective treatment and rehabilitation
The articles in the evidence map are presented based on included population and intervention. You can filter which articles are displayed by making selections in the menu above the table. Below the table are functions for exporting the selection as an Excel file or image.
| 1 Moderate risk of bias; 2 Few participants, few events; 3 The results have not been repeated; 4 Moderate number of participants. MWD = Minute walking distance; MWT = Minute walk test; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs control | Number of participants (Number of studies and study design) [Reference] |
Outcome | Certainty of results Reason for reduced certainty |
| Telerehabilitation vs short teaching instructions | 120 (1 RCT) [12] |
Walking distance 6 minutes (6MWD) | Very low Risk of bias −11 Precision −12 Transferability −13 |
| Inspiratory muscle training vs usual care | 281 (1 RCT) [9] |
Health-related quality of life (three domains: psychological, shortness of breath and activity, chest symptoms) | Very low Risk of bias −11 Precision −14 Transferability −13 |
| Instructor-led respiratory exercises via telemedicine vs a brochure describing the same respiratory exercises | 52 (1 RCT) [10] |
Spirometry, walking distance 6 minutes (6MWT) | Very low Risk of bias −11 Precision −22 Transferability −13 |
| Guided breathing training using singing techniques (online) vs usual care | 150 (1 RCT) [11] |
Health-related quality of life | Very low Risk of bias −11 Precision −1 2 Transferability −13 |
| 1 Moderate risk of bias; 2 Few participants, few events; 3 The results have not been repeated. Net = Narrative exposure therapy; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs control | Number of participants (Number of studies and study design) [Reference] |
Outcome | Certainty of results Reason for reduced certainty |
| Narrative exposure therapy (net) & personalized psychological treatment vs personalized psychological treatment | 111 (1 RCT) [21] |
Post traumatic stress | Very low Risk of bias −11 Precision −12 Transferability −13 |
| 1 Moderate risk of bias; 2 Few participants, few events; 3 The results have not been repeated; 4 The results have only been repeated as pilot study on the same research group. NRSI = Non-randomised studies of interventions; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs control | Number of participants (Number of studies and study design) [Reference] |
Outcome | Certainty of results Reason for reduced certainty |
| Palmitoylethanolamide and Luteolin (orally) combined with smell training vs smell training | 185 + 12 participants (2 RCT) [16] [17] |
Smell function | Very low Risk of bias −11 Precision −12 Transferability −14 |
| Corticosteroids (methylprednisolone) combined with smell training vs smell training | 27 participants (1 NRSI prospective) [18] |
Smell function | Very low Risk of bias −11 Precision −22 Transferability −13 |
| 1 Moderate risk of bias; 2 Few participants, few events; 3 The results have not been repeated; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs control | Number of participants (Number of studies and study design) [Reference] |
Outcome | Certainty of results Reason for reduced certainty |
| Acetyl-L carnitine combined with rehabilitation training vs rehabilitation training | 60 (1 RCT) [22] |
Experienced pain and shortness of breath | Very low Risk of bias −11 Precision −22 Transferability −13 |
| 1 Moderate risk of bias; 2 Few participants, few events; 3 The results have not been repeated; NRSI = Non-randomised studies of interventions; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs control | Number of participants (Number of studies and study design) [Reference] |
Outcome | Certainty of results Reason for reduced certainty |
| Cognitive training vs no treatment | 45 (1 NRSI) [20] |
Cognitive function | Very low Risk of bias −11 Precision −22 Transferability −13 |
| 1 Moderate risk of bias; 2 Few participants, few events; 3 The results have not been repeated, and the population other than from a Swedish context, mainly the diagnosis ’qi deficiency’. MWD = Minute walking distance; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs control | Number of participants (Number of studies and study design) [Reference] |
Outcome | Certainty of results Reason for reduced certainty |
| Chinese herbal medicine (Bufei Huoxue) vs placebo | 131 (1 RCT) [23] |
Lung changes (CT scan), walking distance 6 min (6MWD) | Very low Risk of bias −11 Precision −12 Transferability −23 |
Discussion
All in all, the scientific basis has very low reliability. It is therefore not possible to assess whether any of the treatments studied are effective or not, on the basis of the evidence identified up to and including 1 June 2022. This does not mean that the treatments have no effect, but that more well-done studies are needed to assess the effect.
Conflicts of interest
In accordance with SBU’s requirements, the experts participating in this project have submitted statements about conflicts of interest. These documents are available at SBU’s secretariat. SBU has determined that the conditions described in the submissions are compatible with SBU’s requirements for objectivity and impartiality.
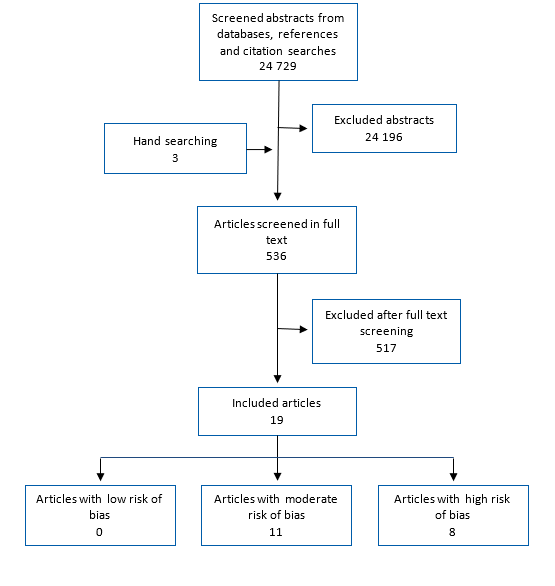
Flow diagram for article screening
Evidence map: Post COVID-19 – effective treatment and rehabilitation
The articles in the evidence map are presented based on included population and intervention. You can filter which articles are displayed by making selections in the menu above the table. Below the table are functions for exporting the selection as an Excel file or image.
Project group
Experts
- Alison Godbolt, Danderyd Hospital
- Judith Bruchfeld, Karolinska University Hospital
- Jörgen Månsson, University of Gothenburg
- Kristina Hedman, Sundsvall Regional Hospital
- Marcus Ståhlberg, Karolinska University Hospital
- Michael Runold, Karolinska University Hospital
- Olof Hertting, Astrid Lindgren Children's Hospital
From SBU
- Elizabeth Åhsberg, Project Manager
- Nathalie Peira, Assistant Project Manager
- Idha Kurtsdotter, Assistant Project Manager (from April 2022)
- Jessica Dagerhamn, Assistan Project Manager (from November 2021 to April 2022)
- André Sjöberg, Assistant Project Manager (up to December 2022)
- Maria Ahlberg, Project Administrator
- Carl Gornitzki, Information Specialist (up to November 2021)
- Hanna Olofsson, Information Specialist (from December 2021)
- Pernilla Östlund, Head of Department
- Susanne Eksell, Web Project Manager
- Irene Edebert, acting Head of Department (from November 2021)
More on the subject
SBU and COVID-19
SBU closely monitors what actions other countries are taking to meet the needs of decision- and policymakers to respond to the COVID-19 pandemic. Via the International organisation of HTA, INAHTA, SBU shares and exchanges information to support staff and decision-makers in health care and social services.
SBU is also in contact with the other Swedish agencies within the national health care sector who currently are working intensively to support both the health care services as well as care homes.

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email